Executing a Successful Remediation Program: Part 2 of 3

The second part of our three-part series on executing an effective remediation program starts by looking at primary versus secondary root causes. A robust quality system provides an assurance of high-quality production efforts and products. At NSF, we recommend that a systems-based approach to remediation and corrective action is the most successful approach. Also, a system- based approach is recognized globally with, regulators such as the MHRA and FDA.
Don’t Just Look for a Quick Fix
This is the point in the program at which a review of your systems will be used in conjunction with your strategy and risk-based evaluation to ensure that the remediation program is not just focused on skimming the surface and assigning corrections to top level issues without a review of the underlying supporting systems. For instance, consider one of the most common observations from FDA over the past few years, which is that investigations are not being performed in a thorough manner, or that they potentially don't reach root cause and that they're found to be ineffective. Hence, they either have an overall high number of investigations, perhaps, or maybe they have a very high rate of repeating deviations. At first glance a remediation effort might simply perform a quick retraining of investigators and then just get back to work or perhaps hire outside assistance to drive down many open or overdue investigations. This approach is likely to fail to solve the problem in a long-term sustainable manner. It sounds simple but sometimes in the heat of a regulatory response the underlying systems can get lost in the shuffle of that immediate action.
Taking an approach, such as the one outlined above, can easily lead to issues with supportive systems being overlooked. Supportive systems in this case include good investigations, robust training evaluation, or internal audits.
Ask These Questions
Consider these questions, when looking at supportive systems that might have been overlooked in the heat of the early stages of a remediation program:
- Has there been a budget that's been supplied for these changes?
- If additional inspection programs have been created, have they been successful and have processes been properly validated or revalidated after changes are made?
- Is your change control programming just actually functioning at an appropriate level or has it just become a paper exercise?
These are just some of the supporting systems that must be evaluated as part of the remediation before we jump in start the remediation program.
What Is the Goal of Remediation?
Every company wants to stay compliant
A company that prides itself on only being compliant is failing to capitalize on the other business advantages of a robust quality system. A fully functioning quality system will provide assurance that the products being manufactured are of high quality and will help to maintain a state of compliance.
What outcome do companies want from a remediation program? They want it to transform the quality systems that are already in place and to create operations that are in a constant state of inspection readiness. Being in a constant state of inspection readiness state will resolve the immediate concerns highlighted by a regulatory authority and create a sustainable compliance program centered on quality.
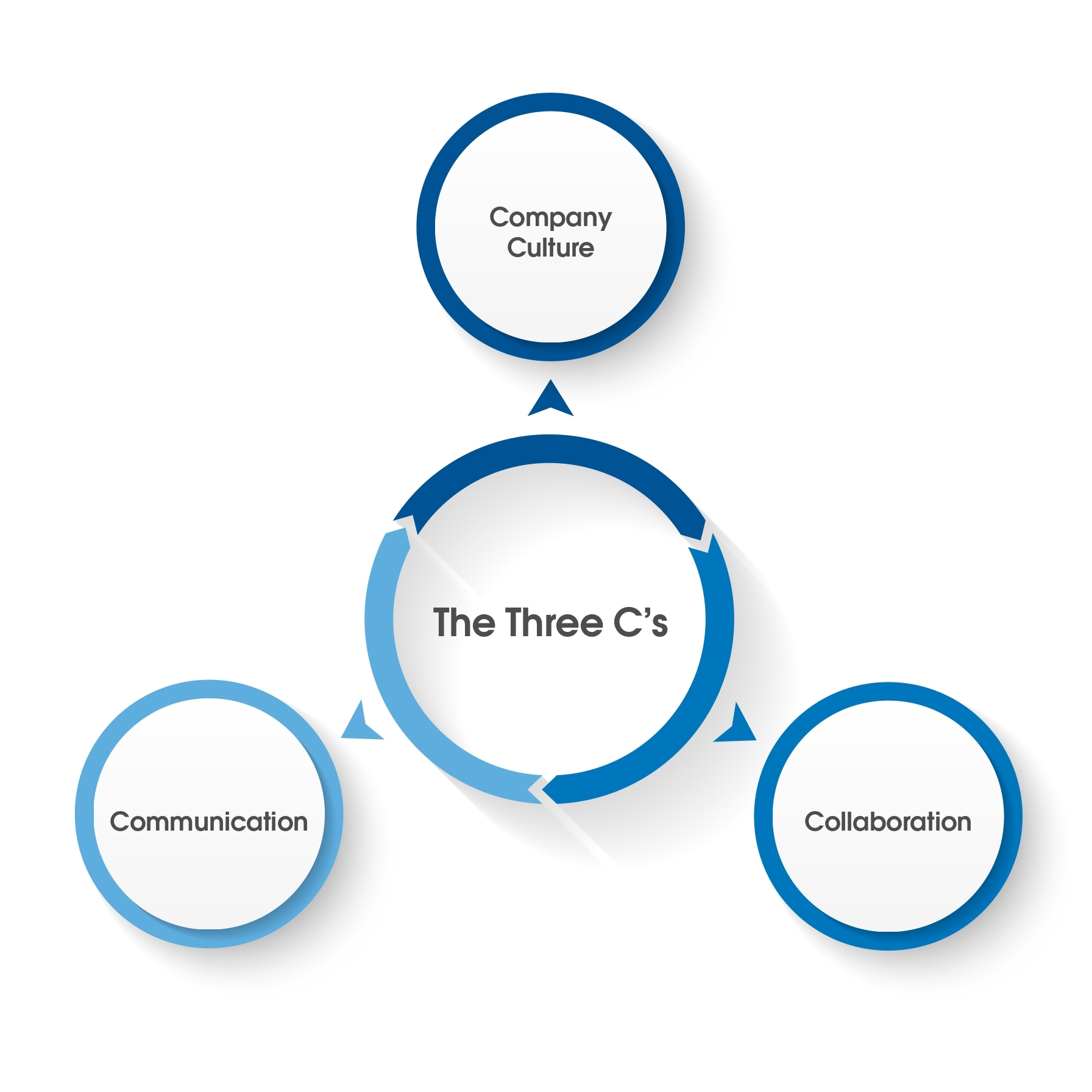
The Three C’s
The NSF team of experts has reflected on the projects that we have managed for client companies and has come to understand that a remediation plan that uses the points discussed above and in part one of the series will require the three C’s:
- company culture
- collaboration
- communication
A Quality Culture
The three factors encapsulate the critical elements of a successful remediation program. It is useful to think of these concepts as part of a three-legged stool. While a team approach oftentimes will include communication and collaboration, the additional effort must be made generally by the management group to ensure that the value proposition and belief in the activities being executed are passed down to all the divisions or the groups and the individuals that participate. This is made more critical if the target is a global company and has multiple facilities around the world. A management group must lead by example to avoid conflicts with the ‘commitment to action’ versus current behavior. Such conflict sends a clear message to the rest of the organization that some behaviors are still being tolerated and possibly rewarded even if they run contrary to the remediation strategy.
Many people talk about the concept of a quality culture. What do we mean by this? Essentially, it is an unseen desire and commitment to evaluate the organization and its processes and then try to find ways to do business that are both simpler as well as more productive. Adopting the mindset of a quality culture increases the chances that compliance will fall into line, which will lead to long-term successes rather than a ‘picture of quality’ that only exists in one singular moment.
The Importance of Management and Quality Teams Being in Sync – Collaboration
Our experience has shown us that the priorities/values that are promoted and reflected in the organization’s support systems and personnel will ultimately influence the way the remediation activity is executed. Simply put - do what I do.
When the management group is not in sync with the vision of the plan and the principles of the solution it becomes much more difficult to change the behaviors of the supporting personnel. Therefore, to succeed we need to underpin the remediation program with a culture of quality that exists in real life, and not just on a sheet of paper. Also, when the people working tirelessly to execute a plan see that the management group is providing active support for a change in culture and behavior, that will give momentum to the project.
The Need for a Steering Committee or Oversight Team
A meaningful way to demonstrate management commitment to the program is to establish a steering committee or an executive oversight team. Regardless of the terminology, when a group is in place that consists of senior managers and executives, provides three important gains for the process:
- It allows for a direct and frequent place to raise critical issues or roadblocks.
- It allows for a regular meeting of managers that represent the various functional areas of the company and it provides them a place to get together and recap the activity between meetings to help alleviate any crossover concerns or pinch points that might impact multiple groups.
- Most importantly in this case, it demonstrates that the executive group is really embedded in the program itself and they're taking time out of their day to commit to, listen and provide feedback on the process which speaks to our concept of communication and culture being critical elements of a successful program.
The oversight team is going to become the place where the vision is set, resources are provided, and decisions are made.
Talk to Our Experts Today
The NSF Pharma Biotech consulting and training team consists of ex-regulators and industry experts who have extensive experience of assisting companies with remediation programs. They have worked on projects with companies of all sizes all over the world.
If you have an issue that you feel that our team can assist you and your company with, then complete the form below and one of our team members will be back to you to arrange an initial call to see how we can help.
Would You Like To Learn More About Executing a Successful Remediation Program
Contact us with questions or to receive a quote.
Resources
How NSF Can Help You
Get in touch to find out how we can help you and your business thrive.

What’s New with NSF

Brooklands New Media’s Publication On NSF’s Global Animal Wellness Standards (GAWS) Not Endorsed by NSF
November 25, 2024
NSF Opens Advanced Water Testing Laboratory in Germany
October 17, 2024
World Food Day: A Global Call to Action for Food Security and Sustainability
October 16, 2024


