Executing a Successful Remediation Program: Part 1 of 3

We have performed both large and small remediation programs around the world and have brought companies from a very difficult position to one that is not only compliant but focused on quality, with the ability to maintain their systems over the long term in a sustainable and efficient manner.
Our experts have performed thousands of inspections around the globe and understand the need to leverage company culture and communication to help drive the corrections needed to ensure successful remediation efforts can be realized.
This multi-part article will look at the following:
- Why a company needs to initiate a remediation program after a regulatory inspection.
- What the intended outcomes of any program are going to be and what they should be.
- We will also look at what should be considered prior to starting remediation work.
- The key factors to consider before you embark on a remediation project or take any actions.
We will then move into what is one of the most critical parts of the process which is to ensure that:
- Objectives can be met,
- That they are sustainable
- That corrections put in place are appropriate for the issues at hand
- That they do not fall too short or become too burdensome and unnecessary to accomplish the task.
At NSF, we have three C’s that we associate with remediation. These are the critical success factors necessary for any remediation program. They cover everything from the initial alert to the final effectiveness checks. We will also consider the questions that an organization needs to be asking itself, and we will end with a list of six common obstacles that we encounter when companies attempt to drive changes.
When Do You Need a Need a Remediation Program?
There are two reasons why an organization will require a remediation program. The first is when a deficiency or risk on a currently established quality systems has been identified.
This is the better situation to be in because it shows that the systems in place are functioning at a level that is sufficient to identify risks and escalate them in an appropriate manner.
What is also good about this kind of program is that at least it is not usually an immediate surprise or shock to the company. The ability to contain the project and any response to within the company domain means that there are going to be fewer external pressures or stresses when it comes to reporting to a regulatory body or to meeting any other externally created timelines.
The second source of a remediation is a deficiency that has been identified by an external source. This might include supplier audits or customer audits. However, the second source is more generally linked to a regulatory inspection. This is the most common reason that a program is started, and it can bring large amounts of stress on a company when it is potentially facing a ‘laundry list’ of observations. When this happens, companies need to react with energy and purpose.
Lifecycle of a Remediation Program
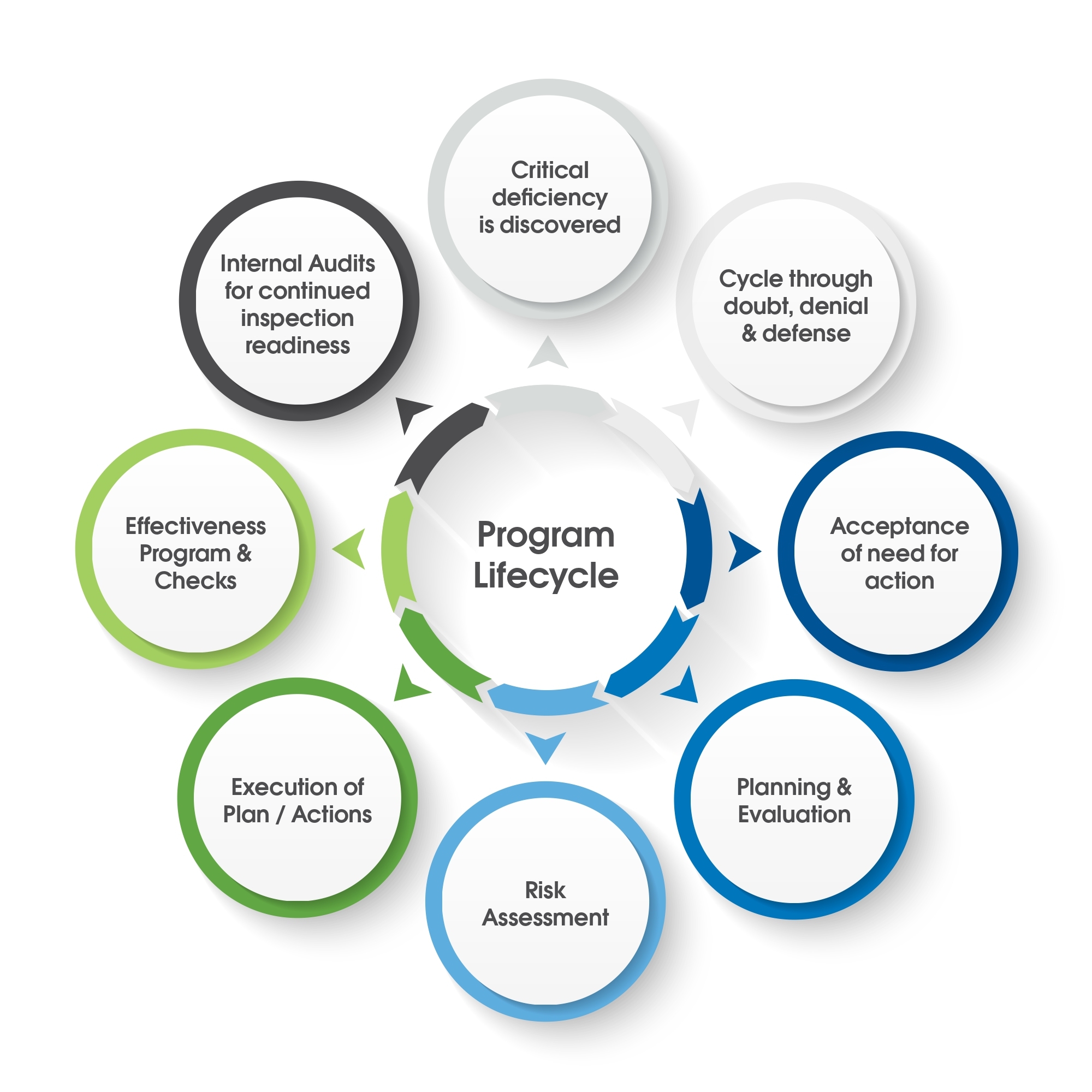
What Happens When Issues Are Identified?
Typically, external identification of issues, usually by a regulatory agency upends the best made plans of any company. With the list of observations come tight timelines, the need to allocate resources to deal with the issues, when these have usually not been budgeted for.
What happens in the immediate aftershock of learning that remediation is required? Firstly, the company will need to assess the gravity and enormity of the situation. Depending on the scope of the observations, this will include considering the short, mid-term, and long-term implications for the company, depending on how serious the deviations are.
Do Not Jump to Conclusions
It is important not to read a document such as a Form 483 observation word for word and then to instantly jump to a conclusion for the path forward. That is a reactionary approach. At times like this, many of our experts recommend that people refer to the guidance for ICH Q9 for risk management to help drive priorities such as “what that initial response is going to look like?”
A problem that we see with industry repeatedly is when a management group looks through an inspection report and call for immediate action without sufficient strategy or planning. This approach usually leads to failures in subsequent inspections for the very same issue if not very similar issues. This can quickly become a repetitive cycle of failure. It is a term that we like to call ‘chronic quality system non- compliance.’ There is no doubt that people in those positions who take that approach have the best intentions. However, that approach only scrapes the surface and drives uninformed management choices, and it can become a very difficult cycle to break.
The Role of Management in a Remediation Program
For a remediation program to be effective, management need to buy into two things. Firstly, this is a very serious situation which is going to require a large amount of their attention. Secondly, they need to fully buy into whatever plan is being put together. It is not sufficient to simply inform the executive group about specific items for your plan such as overall cost or timeline and then walk away to execute. A company’s management team needs to understand what needs to be corrected, and why it needs to be corrected.
What we have found in our many years of leading remediation programs of all sizes, is that there is an element of cultural change required for serious change action to be enacted and assimilated into the company. A cultural shift will normally have a much higher likelihood of being successful if it is driven from the top down and embraced as the new paradigm going forward. If not, the business-as-usual model will be triumphant, and change will not come about. Right at this moment in the project, there is the danger of a fracturing of the culture and the team causing some people to seek to maintain the status quo, while others seek change. This can have damaging consequences for the program itself.
Do Not Go Into Denial
Human nature, and ‘it cannot be happening to me/us’ is a very common reaction among executives and quality professionals at a time like this. Deflection and blame can be dominant actions. “It was an inexperienced inspector,” or “this was an overzealous audit” are common feelings and statements. Bargaining also plays a part here, as company executives attempt to diminish the extent of the problems, and thus the ramifications for their manufacturing plant. Again, this does not work, and it is not a ‘way out.’ Sticking plaster solutions will only lead to a cycle of continuous failure in subsequent inspections. Once we get past denial and eventually to acceptance, we need to quickly create a high-level strategy for the program.
High-Level Strategy for a Remediation Program
This kicks off with essential questions:
- What is it going to look like?
- Who is going to lead it?
- How long is it going to take?
- How much money is it going to cost?
- Is it going to interrupt current business continuity, or will operations be able to continue concurrently?
These are just some items that need to be discussed amongst the management group as well as company leaders. At this point the company is under pressure to meet required time frames to respond to observations. At this point, the company needs to consider if they can do things themselves or bring in a specialist consulting company that has remediation subject matter experts assist with both the response itself as well as strategy planning. A strategic, well-planned remediation is paramount to long term sustainable corrections and corrective actions.
Part Two of ‘Executing a Successful Remediation Program’
The second part of this three-part series looks at the following:
- Primary and secondary root cause
- Reviewing your systems
- The business benefits of a robust quality system
- And more
Read part two here, or skip ahead now to part three here.
Talk to Our Experts Today
The NSF Pharma Biotech consulting and training team consists of ex-regulators and industry experts who have extensive experience of assisting companies with remediation programs. They have worked on projects with companies of all sizes all over the world.
If you have an issue that you feel that our team can assist you and your company with, then complete the form below and one of our team members will be back to you to arrange an initial call to see how we can help.
Would You Like To Learn More About Executing a Successful Remediation Program
Contact us with questions or to receive a quote.
Resources
How NSF Can Help You
Get in touch to find out how we can help you and your business thrive.

What’s New with NSF

GMP and Regulatory Compliance Virtual SupplySide Connect New Jersey Training
January 30, 2025
NSF Granted Reauthorization as a CMMC Third-Party Assessment Organization
January 8, 2025
Sustainable Foods Summit 2025
January 2, 2025


